Atmosphere Composition, Layers, & Human Impacts


Atmosphere Objectives:
- Describe the basic composition of Earth’s atmosphere, including how atmospheric gases relate to ozone depletion and acid rain.
- Explain the greenhouse effect including how it relates to Earth’s climate.
- Provide examples of how light and color relate to leaf appearance and function.
Now that you have gone through the basics of the course, including developing a safety checklist, this module introduces what to look for in the environments you may encounter.
In this guide you will be building a field kit from materials you have around you (creative improvising and up-cycling encouraged!). In the next guide you will have your first field assignment, and it can be completed indoors successfully, if needed.

In Guide 1A, we went through the hierarchy of life from atom to biosphere.
We are starting this Module with the parts of the biosphere.
The biosphere consists of three components. What are they?
This video models a miniature biosphere you can make yourself.
If you would like to make your own biosphere, information is available on the resources page.
Now we are focusing in on the basic characteristics of Earth’s atmosphere.
This view of Mount Hood is from a jet traveling five miles above Earth’s surface, near the top of the troposphere.
Although we breath in nitrogen and need it for cellular structure and function, it takes bacteria in the soil and water to convert atmospheric nitrogen onto a form other organisms can utilize.
Atmosphere Basics
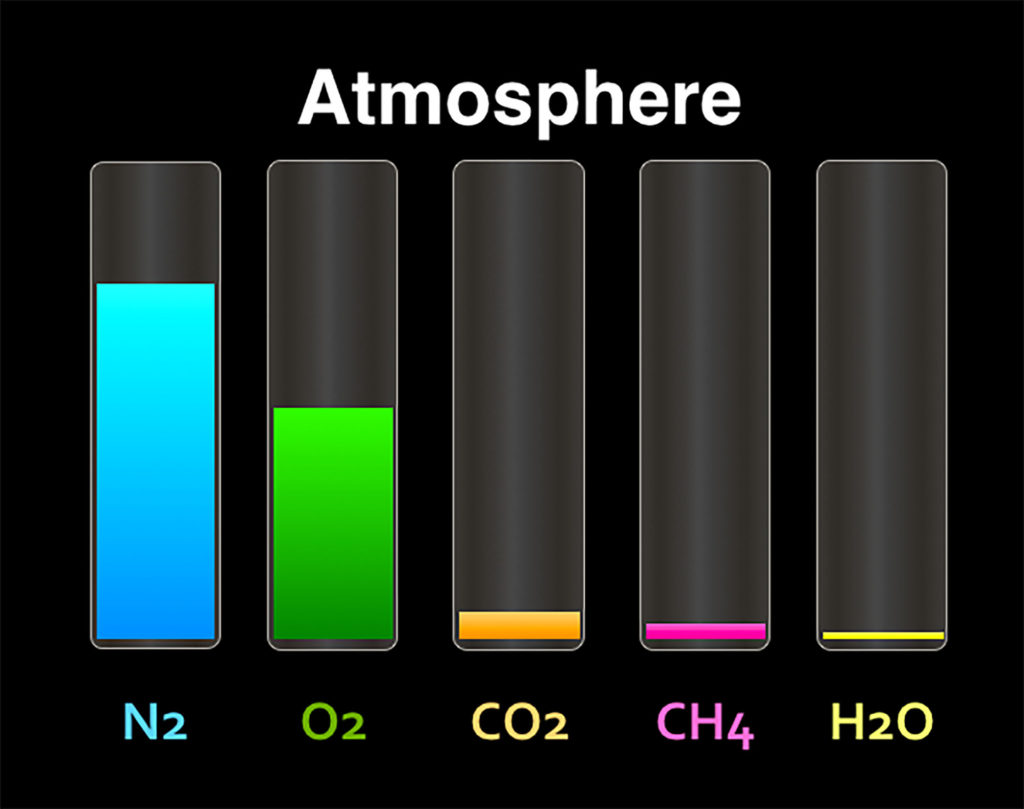
Many of these gases are not directly utilized by humans, although we may need some of the nutrients (like nitrogen) to eventually reach us through the food web. Which gas can we directly utilize?
The Earth has a layered atmosphere, with different densities and composition of gases.
For example, there is a higher amount of ozone in part of the stratosphere than in other layers.

From the illustration above, which atmospheric layer do humans primarily live within?
Human impacts on the atmosphere

Chlorofluorocarbons (CFCs) were thought to be relatively harmless molecules, and were used as refrigerants and propellants (in things like cans of hairspray).
CFCs entered the stratosphere and reacted with the ozone molecules, tearing them apart. It turned out that ozone was reducing the amount of ultraviolet light that reached the earth’s surface.
Ultraviolet light can cause mutations, and increased levels impacted a wide range of organisms. In humans, the areas most impacted by ozone depletion (for example in New Zealand), has higher rates of cataracts (vision loss) and skin cancer.


The Montreal Protocol of 1987 limited CFC production, and although CFCs remain in the atmosphere for decades, they are decreasing and ozone concentration is recovering.
This is considered to be one of the most impactful international environmental treaties ever enacted.

Which two molecules can react with water to form acid rain? _____ and _____
These molecules are produced by natural sources like wildland fires and volcanic eruptions. Human activities release concentrated streams of these gases into the atmosphere, where they can interact with water vapor.
Acid rain is also called acid deposition because it can be acid fog, acid snow, or acid sleet as well. What are two possible impacts of acid rain?

This video contextualizes acid rain in comparison to better-known household products.
Greenhouse Effect & Climate
Think about how many times during the week that you have discussed the weather with someone else, or how many times you changed clothes or plans related to the weather.
Weather is short-term trends in temperature and precipitation; climate is a long-term trends in accumulated weather events.

Specific weather can be difficult to predict more than a week in advance, and that gives an indication of how challenging it is to create long-term climate models.
Instead of specifically predicting a day’s temperature or a week’s precipitation, climate models plot anticipated trends based on accumulated weather events over time.
This video introduces the greenhouse effect.
Our climate is created by atmospheric gases that retain heat. What are some of these greenhouse gases?
Too high of a concentration of greenhouse gases leads towards an atmosphere more like Venus; too low of a concentration is more like Mars.

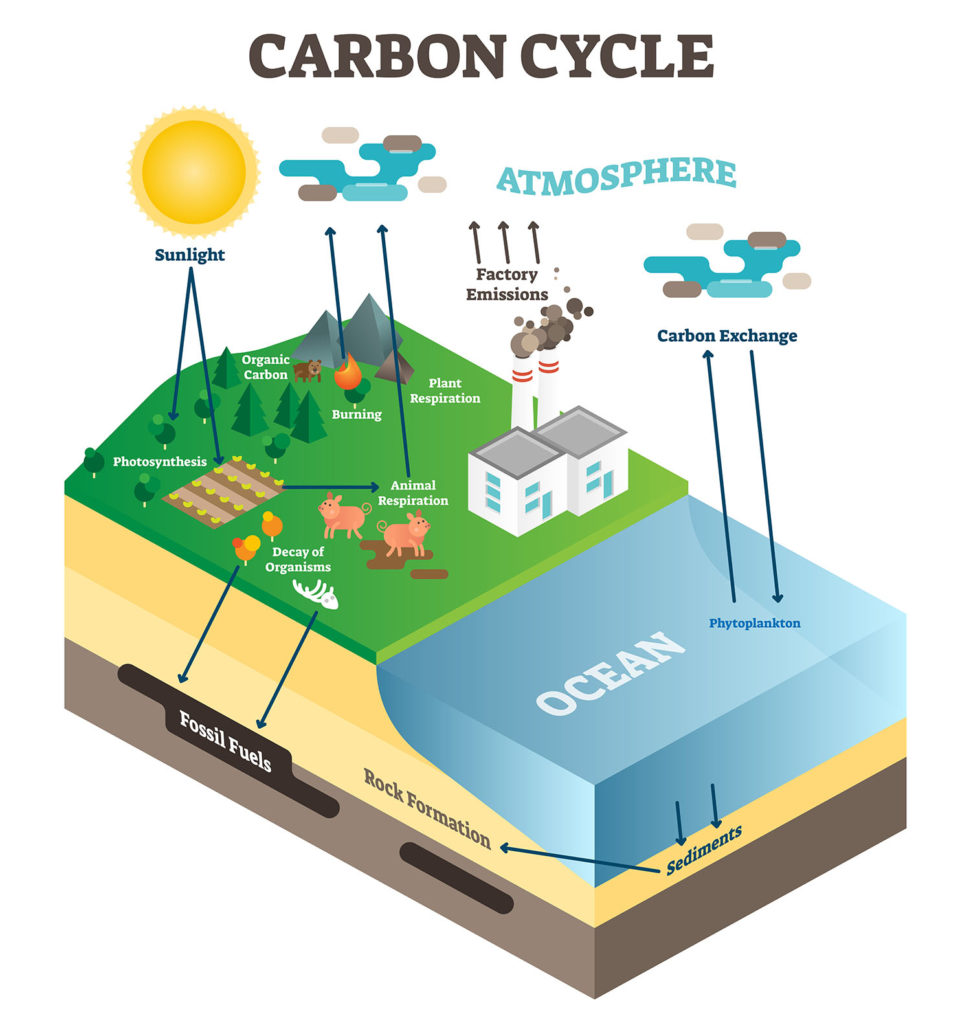
This figure indicates some of the human activities that can increase the amount of greenhouse gases in the atmosphere.
We’ll have more on how climate change and its impact on ecosystems in upcoming guides.
Sunlight, Color, & Leaves

When white light passes through a glass prism, we see a rainbow of colors with visible (to humans) wavelengths.

The color we see is reflected off, not absorbed by, an object. If we see a green leaf, the green wavelengths were reflected off of the plant.
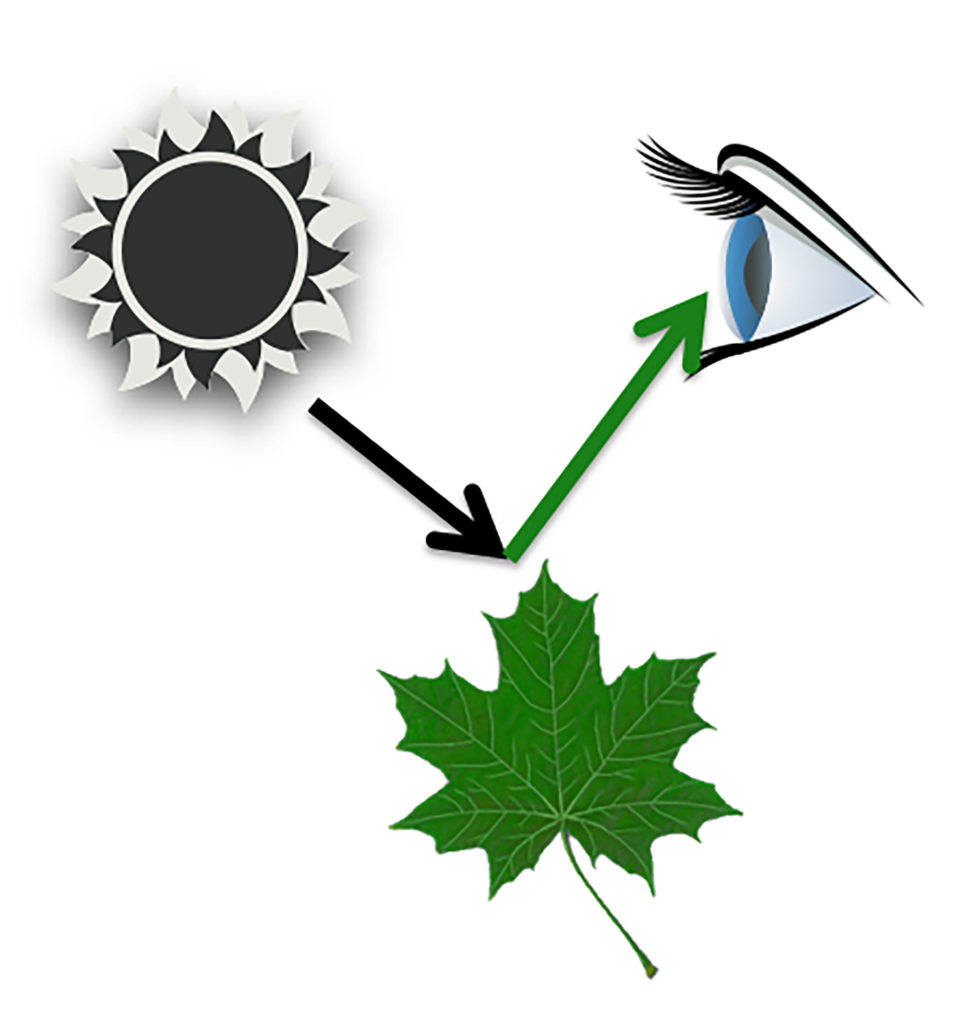

When you see a spinach leaf, the green color comes from the dominant green chlorophyll pigments, but other color pigments are present.
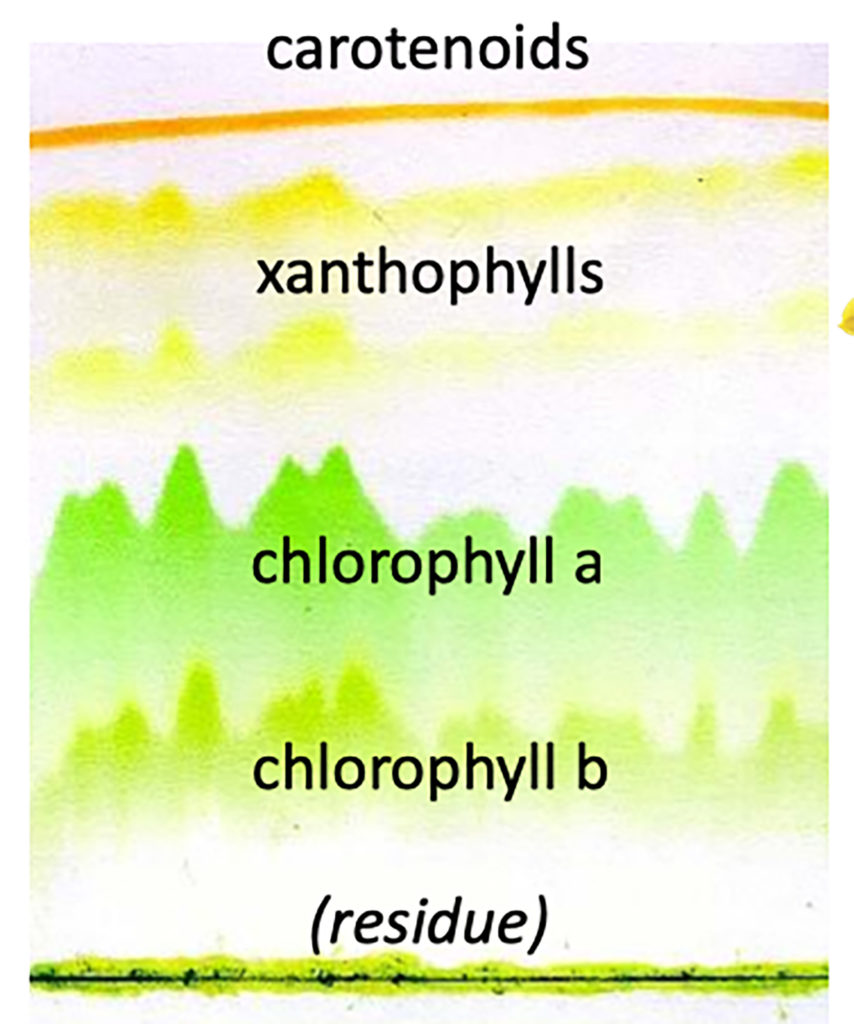

Fall leaf colors occur when chlorophyll degrades as winter approaches, day length shortens, and temperatures cool. Loss of chlorophyll reveals yellow, orange, and red pigments (carotenoids and xanthophylls) that were always there, but in lesser amounts than the green chlorophylls.

Producers high in Carotenoids

Consumers who eat them
Leaf color can relate to light intensity.
We have all experienced how much cooler it can be in the shade of trees. Reduced sunlight can result in a small habitat (microhabitat) with increased moisture and lower temperature.
The next section introduces the hydrosphere, including the significance of the water molecule to biological functions.

Check your knowledge. Can you:
- Describe the basic composition of Earth’s atmosphere, including how atmospheric gases relate to ozone depletion and acid rain?
- Explain the greenhouse effect including how it relates to Earth’s climate?
- Provide examples of how light and color relate to leaf appearance and function?